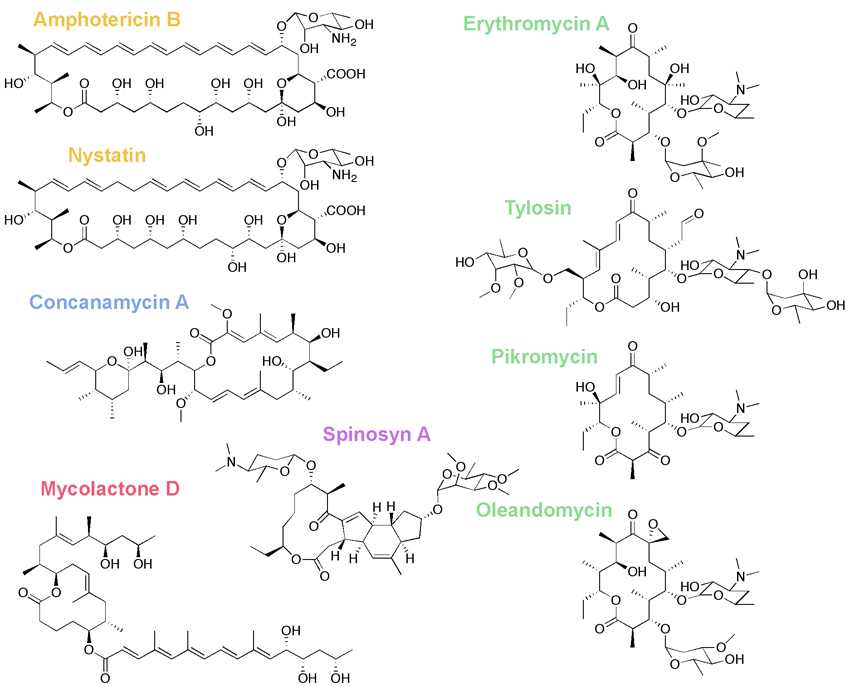
Many important pharmaceuticals, including the antibiotic Erythromycin and the immunosuppressant Rapamycin, belong to a diverse class of molecules called polyketides. These complex molecules are synthesized by modular polyketide synthases (PKSs) - enormous enzymes that are directly analogous to assembly lines. Our group seeks to understand this chemical machinery and engineer it to produce new molecules and new medicines.
Biosynthesis
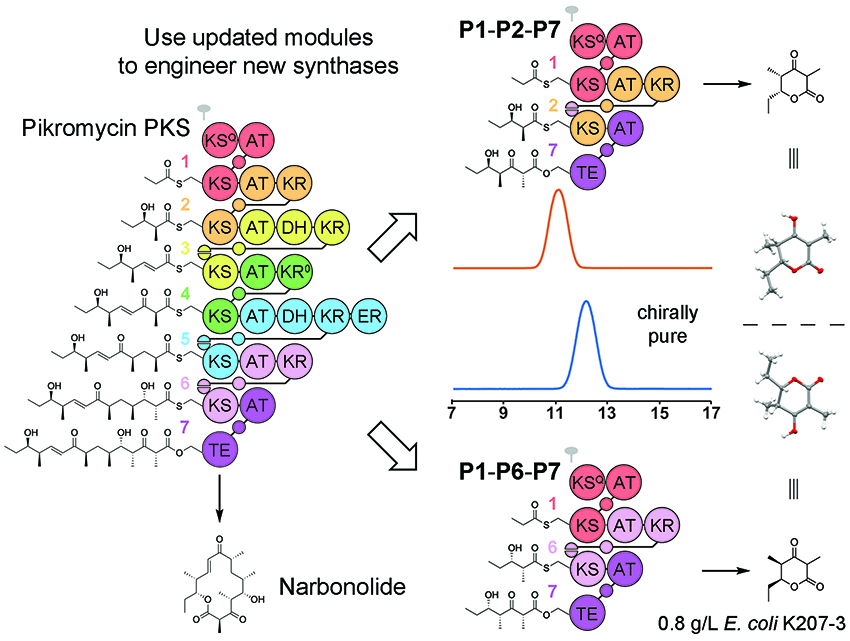
Each enzyme within these megasynthases operates on a polyketide only once during its synthesis. Transformations catalyzed by PKS enzymes include carbon-carbon bond formation, cyclizations, and stereocontrolled reductions and eliminations. Our research attempts to determine the mechanisms and specificities of each enzyme type in order to genetically engineer PKSs that produce derivatives of known polyketides and libraries of completely novel polyketides.
Biophysics
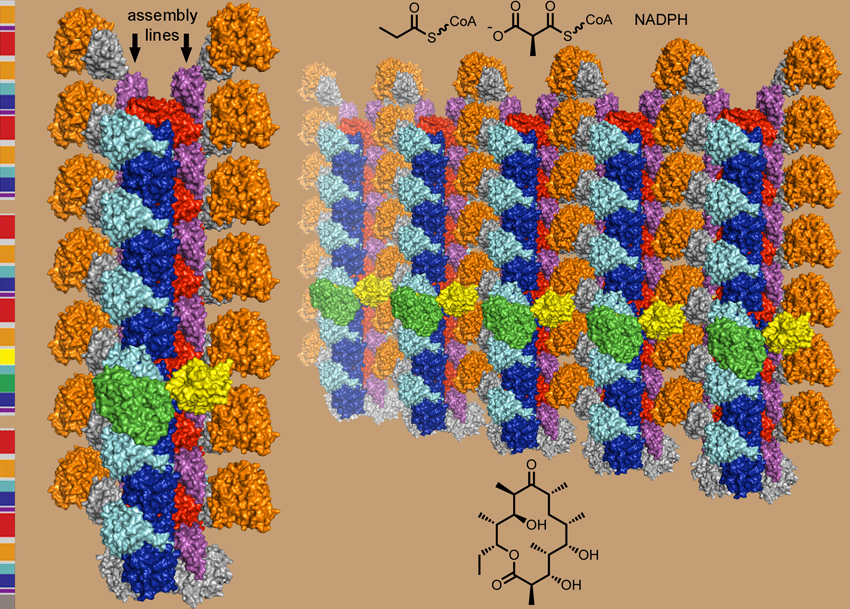
The domain boundaries of enzymes within PKSs have been identified, enabling structural studies of isolated domains, sets of domains that form modules, and sets of modules that form synthases. Through crystallography, electron microscopy, and other structural methods, we are working towards an atomic level understanding of PKS assembly line architecture and dynamics.
Biocatalysis
PKSs are the enzymatic champions of organic synthesis, performing complex, stereocontrolled reactions on diverse carbon chains. Our lab is learning how to harness the catalytic potential of PKSs and utilize them to perform desirable chemical transformations and generate designer polyketides. As PKS biocatalysts are catalytically active under ambient conditions in an aqueous environment, they can be considered a new paradigm in "green chemistry."
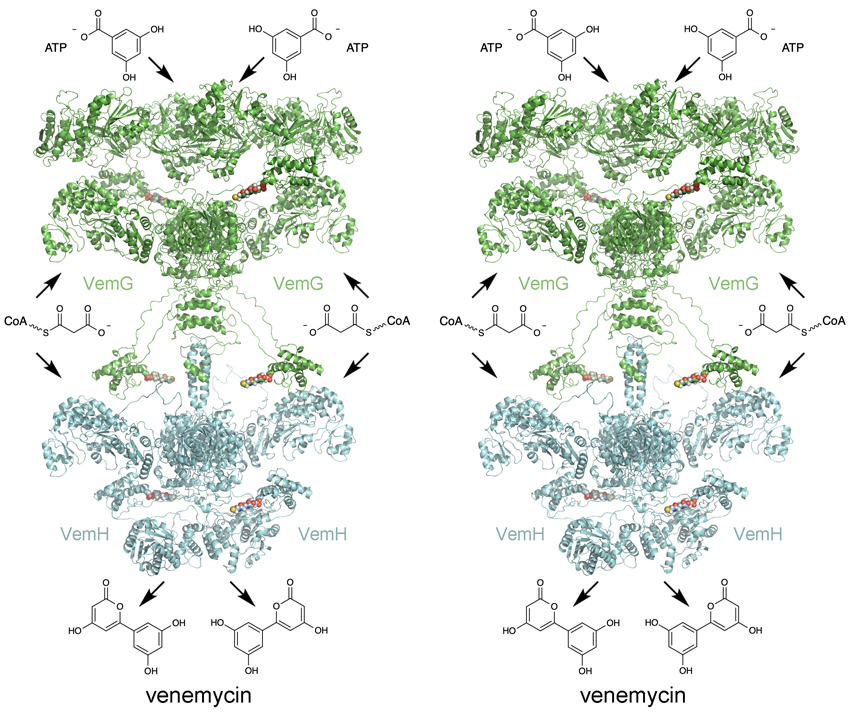 Download a rough, all-atom, .pdb model of the venemycin PKS
Download a rough, all-atom, .pdb model of the venemycin PKS



